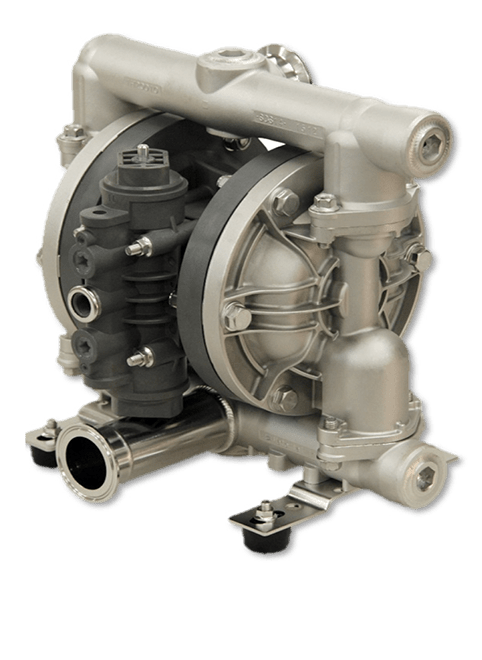
FDA Compliant Diaphragm Pumps
FDA Approved AODD Pumps are manufactured with polished stainless steel wetted parts and FDA compliant materials & sanitary flange fittings. Commonly used in food, beverage, cosmetic, pharmaceutical & chemical applications where 3A, USDA and EHEDG certification are NOT required. For decades, Iwaki Air has been the go-to choice for food processing industries looking for highly polished surface finishes. Our FDA approved pumps provide great cleanability, ease of maintenance, and dependability for your specialized hygienic applications. These air-operated double-diaphragm (AODD) pumps are manufactured with FDA compliant components and can be used in food processing applications.
Key Features of FDA Diaphragm Pumps
- 100% Pneumatic Operation
- Can Handle Large Solids Sizes & High Flow Rates
- Welded & Oversized Sanitary Flange Connections
- Electro Polished Stainless Steel Wetted Section & Hardware
- FDA Compliant Diaphragms, Check Valves, Seats, Guides & Seals
- Bolted Construction with Machined Mating Surface
- Outside Accessible Looped C® Air Motor
- Compact & Robust Construction
- Common “Drop-in” Pump Dimensions with Adjustable Footprint
- 100% Non-Lubricated Air Motor
- Fully Torqued and Tested Prior to Shipment
- Engineered for High Performance Operation & Reliability
FDA Pumps are available in several Sizes & Materials
- ¼”, 3/8”, ½”, ¾”, 1”, 1 ½” 2” & 3” Stainless Steel Pump Models
- Shipped with Oversized Sanitary Flange Connections
- Electro Polished Stainless Steel Body and Hardware
- FDA Compliant PTFE Elastomers, Liquid Seals & Check Valves
- Optional PPS, PPG or PTFE Coated Aluminium Air Motor Section
- # ISO Sanitary Flanges Shipped as standard with Optional 3A also available
- # FDA Compliant Drum Pumps & FDA Pulsation Dampeners are also available
What is FDA Compliant?
FDA compliance refers to the process in which 3A Sanitary and USDA Dairy Division Standards are not required by the United States Food and Drug Administration (FDA). Pumping the completed product requires 3A or USDA pumps, however this accounts for just a minor fraction of the industry. An FDA-compliant pump can typically be used to introduce materials into the process before the final product is produced.
Iwaki Air pumps are designed and built with FDA-compliant materials and meet certification standards for use in the food, pharmaceutical, and cosmetic industries. These pumps have stainless steel wetted components with a passivated satin finish.
Why is it significant whether a component adheres to FDA standards?
The FDA specifies which materials are safe for use in food and medication production. Along with specific materials, the agency advises temperature ranges, acidity levels, and moisture content to ensure performance. Materials must also be able to withstand the extreme circumstances encountered throughout the sanitization process. FDA compliance assures that equipment does not leach any dangerous substances, even when exposed to hot or acidic materials.
FDA-compliant equipment and parts are crucial because they adhere to established rules for the safe use of materials. To assure compliance and customer safety, the FDA inspects food and medicine production processes on a regular basis. Non-compliance can result in penalties, lawsuits, and even shutdowns.
Important Considerations for FDA Compliant Pumps
Material Composition: The materials used in the construction of the pump must be FDA approved for use in contact with food or pharmaceuticals. Typically, this includes materials such as stainless steel, certain plastics like PTFE (polytetrafluoroethylene), and elastomers that are compatible with FDA regulations.
Hygienic Design: The FDA compliant pump should be designed to minimize areas where product can accumulate and potentially cause contamination. Smooth surfaces, sanitary fittings, and easy disassembly for cleaning are common features of FDA compliant diaphragm pumps.
Sealing: The pump should provide effective sealing to prevent any leakage or contamination of the pumped product. Diaphragm pumps are often chosen for their ability to provide a seal between the wetted components and the environment.
Compliance Documentation: Manufacturers of FDA compliant diaphragm pumps typically provide documentation certifying compliance with FDA regulations. This documentation may include material certifications, compliance statements, and test reports.
Cleaning and Sterilization: FDA compliant pumps should be designed for easy cleaning and sterilization to maintain sanitary conditions. CIP (Clean-In-Place) and SIP (Sterilize-In-Place) capabilities may be important features for certain applications.
Flow Rate and Pressure: Considerations for flow rate and pressure requirements should also be taken into account to ensure the pump meets the specific needs of the application.
Different Applications of FDA Compliant Pumps
FDA compliant pumps are essential in various industries where hygiene, cleanliness, and regulatory compliance are paramount. Some common industries and applications that require FDA approved pumps include:
Food and Beverage: In the food and beverage industry, FDA pumps are used for transferring liquids such as dairy products, beverages, sauces, syrups, and food ingredients. These pumps ensure that the products remain uncontaminated and meet the strict regulatory standards for food safety.
Pharmaceuticals: Pharmaceutical manufacturing requires precise and sterile processes to ensure product safety and efficacy. FDA approved AODD pumps are used for transferring pharmaceutical ingredients, APIs (Active Pharmaceutical Ingredients), and other liquids in a controlled and hygienic manner, adhering to Good Manufacturing Practices (GMP) guidelines.
Biotechnology: In biotechnology and biopharmaceutical industries, FDA compliant pumps play a crucial role in the production of biologics, vaccines, and other biological products. These pumps are utilized for transferring sensitive biomaterials while maintaining sterile conditions and preventing contamination.
Cosmetics and Personal Care Products: Manufacturers of cosmetics and personal care products often use FDA pumps to transfer lotions, creams, shampoos, and other liquid formulations. These pumps ensure that the products remain free from microbial contamination and meet the regulatory requirements for consumer safety.
Medical Devices: FDA approved pumps are utilized in various medical device manufacturing processes, including the production of diagnostic reagents, medical fluids, and pharmaceutical preparations. These pumps help maintain sterility and accuracy in dosage delivery, critical for medical applications.
Nutraceuticals: In the nutraceutical industry, which includes dietary supplements and functional foods, FDA pumps are employed for transferring vitamins, minerals, herbal extracts, and other nutritional ingredients.
Chemical and Petrochemical: Certain chemical and petrochemical applications require FDA compliant pumps for transferring specialty chemicals, additives, and lubricants used in the production of pharmaceuticals, food-grade chemicals, and personal care products.


Get Social